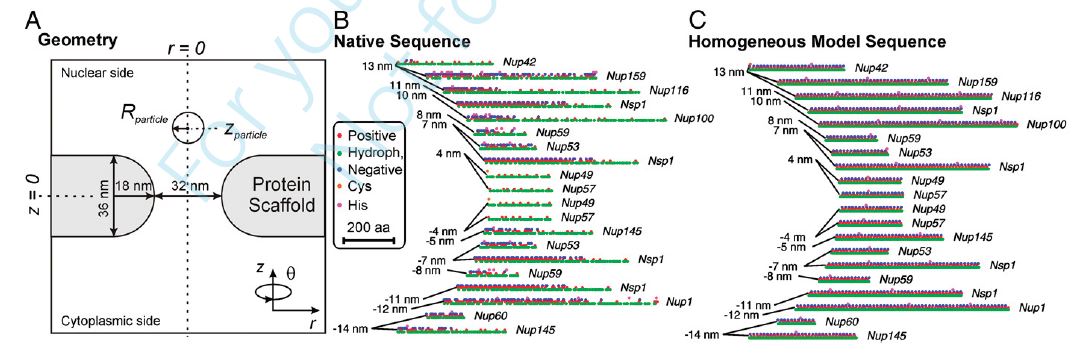
 The molecular structure of the yeast nuclear pore complex (NPC) and the translocation of model particles have been studied with a molecular theory that accounts for the geometry of the pore and the sequence and anchoring position of the unfolded domains of the nucleoporin proteins (the FG-Nups), which control selective transport through the pore. The theory explicitly models the electrostatic, hydrophobic, steric, conformational, and acid-base properties of the FG-Nups. The electrostatic potential within the pore, which arises from the specific charge distribution of the FG-Nups, is predicted to be negative close to pore walls and positive along the pore axis. The positive electrostatic potential facilitates the translocation of negatively charged particles, and the free energy barrier for translocation decreases for increasing particle hydrophobicity. These results agree with the experimental observation that transport receptors that form complexes with hydrophilic/ neutral or positively charged proteins to transport them through the NPC are both hydrophobic and strongly negatively charged. The molecular theory shows that the effects of electrostatic and hydrophobic interactions on the translocating potential are cooperative and nonequivalent due to the interaction-dependent reorganization of the FG-Nups in the presence of the translocating particle. The combination of electrostatic and hydrophobic interactions can give rise to complex translocation potentials displaying a combination of wells and barriers, in contrast to the simple barrier potential observed for a hydrophilic/neutral translocating particle. This work demonstrates the importance of explicitly considering the amino acid sequence and hydrophobic, electrostatic, and steric interactions in understanding the translocation through the NPC. ERRATUM available here »»
for LaTeX users @article{MTagliazucchi2013-110,
author = {M. Tagliazucchi and O. Peleg and M. Kr\"oger and Y. Rabin and I. Szleifer},
title = {Effect of charge, hydrophobicity and sequence of nucleoporins on the translocation of model particles through the nuclear pore complex},
journal = {Proc. Natl. Acad. Sci.},
volume = {110},
pages = {3363-3368},
year = {2013}
}
\bibitem{MTagliazucchi2013-110} M. Tagliazucchi, O. Peleg, M. Kr\"oger, Y. Rabin, I. Szleifer,
Effect of charge, hydrophobicity and sequence of nucleoporins on the translocation of model particles through the nuclear pore complex,
Proc. Natl. Acad. Sci. {\bf 110} (2013) 3363-3368.MTagliazucchi2013-110
M. Tagliazucchi, O. Peleg, M. Kr\"oger, Y. Rabin, I. Szleifer
Effect of charge, hydrophobicity and sequence of nucleoporins on the translocation of model particles through the nuclear pore complex
Proc. Natl. Acad. Sci.,110,2013,3363-3368 |
![]() mk@mat.ethz.ch
1 out of 813 entries requested
[H-factor to-date: > 0]
mk@mat.ethz.ch
1 out of 813 entries requested
[H-factor to-date: > 0]

mk@mat.ethz.ch 1 out of 813 entries requested [H-factor to-date: > 0]